Information for physicians
Basics
The albumin-functionality-test offers a possibility for easy and fast detection of an impairment of the functionality of serum albumin as a transport protein. Pathophysiological changes can influence the functionality. Various modules of the test allow the diagnostics of different diseases like cancer, liver diseases and sepsis.
The approved test module for cancer diagnostics is registered at the German Institute for Medical Documentation and Information (DIMDI) as “other tumor markers”. Diagnostic of sepsis and liver diseases actually takes place within clinical studies.
The Albumin-functionality-test is based on the determination of conformational changes of the albumin molecule. This can be detected by changes in its binding capacity for fatty acids. Addition of different amounts of ethanol induces changes of tertiary structure of albumin molecule in the meaning of an opening. Determination of different diagnostic parameters is possible by analyzing fatty acid binding between these three states (see below).
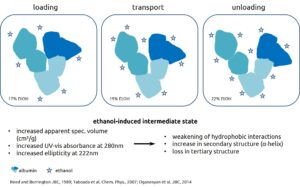
Serum albumin is a transport protein for water-insoluble substances like fatty acids, vitamins and trace elements, as well as metabolites and drugs. Peptide fragments or lipids in blood from tumor cells bind to albumin (Lowenthal et al. 2005, Mehta et al. 2003, Xu et al. 1998) and cause a modification of fatty acid-binding. The same changes are measurable for patients with liver diseases and a heavy toxin burden (Jalan et al., 2009).
These modified binding characteristic can be detected by Electron Spin Resonance Spectroscopy (ESR). A spin labeled fatty acid solved in ethanol is added to the serum and is analyzed by ESR spectroscopy. Status of the binding capacity can be received from analysis of the ESR spectrum of the serum sample.
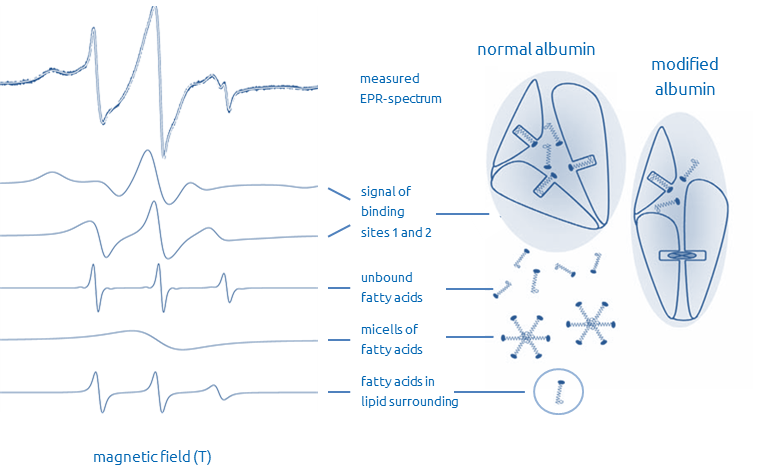
Reporter molecules bind to different albumin binding sites, therefore the ESR spectra shows different components. The figure shows the partition of measured ESR spectrum and resultant binding modes.
Binding constants, binding capacities and biophysical parameters can be determined from the components of the ESR spectrum. Within the cancer module the integral discrimination function (DR – diagnostic result) and transport parameters are calculated, while only specific transport parameters are determined for liver resp. sepsis modules.
Fields of application
The albumin-functionality-test is suitable for therapy monitoring of a known malignant disease, as well as for early detection of a relapse after completion of cancer therapy. Dependent on localization, the Albumin-Functionality-test shows a high specificity (approx. 90%) and a high sensitivity (90%) (Seidel et al. 2005, Kazmierczak et al. 2006, Gurachevsky et al. 2008, Gelos et al. 2010, Moergel et al. 2012).
It is also suitable for early detection of cancer in populations with elevated risk of cancer, like people which are in contact with carcinogenic substances or have an accumulation of cancer in their family history. The albumin-functionality-test so far gives no information about the location of the cancer, therefore it is inadvisable to use it in a screening situation.
The diagnosis of sepsis and liver disease is currently being carried out as part of clinical studies.
Requirements
For an adequate analysis we require:
- At least 2 ml blood stored at 8°C. The whole blood should attain to MedInnovation GmbH within 24 hours after sampling.
- “Pure” (centrifuged) serum should attain to us within at least 2 days after sampling. Centrifugation should be done at 1,000 – 1,500 x g at room temperature. Serum or EDTA plasma must be transferred into a separate and labeled tube.
- Blood drawing systems with anticoagulant (except EDTA) or gel are not allowed and if possible, please don’t use vacuum collection systems.
- For regeneration of serum albumin after surgery, chemo and/or radiation therapy, it is necessary to keep a time lag of four weeks before blood sampling.
- To avoid incorrect test results, please don’t send unchilled blood or serum samples at temperatures above 27°C.
- It is allowed to have a light breakfast.
- Blood donation should be done in the morning.
We can also take a patient’s blood sample in our laboratory in Berlin. Please call us in advance to arrange an appointment.
Acute exacerbations of chronic inflammatory diseases such as Crohn’s disease, ulcerative colitis or rheumatoid arthritis distort test results. For regeneration of serum albumin it is necessary to keep a time lag of four weeks before blood sampling.
After surgery, chemo and/or radiation therapy, it is also necessary to keep a time lag of four weeks before blood sampling. This also applies to diseases with any inflammation.
Unfortunately, it is not possible for us to investigate all medications, whether they might have an influence on the result of the albumin-functionality-test. Long-term medications that cannot be discontinued, should be mentioned before.
At a daily intake of 500 mg or more of Aspirin ® (acetylsalicylic acid) per day, it is necessary for regeneration of serum albumin, to keep a time lag of four weeks before blood sampling.
ASS 100 for hemodilution has no influence.
After intake of anti-inflammation medications (joints, teeth …) for regeneration of serum albumin a time lag of four weeks before blood sampling is necessary.
Interpretation of results
The result of the albumin-functionality-test can be transmitted to you within one day. It consists of the four values DR, BE, RTQ und DTE.
The diagnostic result (DR value) shows a status about the probability of the presence of an active malignant disease. In case of a DR value less than 1.0, an active malignant disease exists or emerges with a high probability. In case of a DR value higher than 1.0 a high probability of no malignant disease exists. In a threshold range from 0.8 to 1.2 is still a high risk of the presence of a malignant disease. This result should be verified after 1 to 3 month by repeating the test.
The further three values called transport parameters, will be considered in case of a DR value in the threshold range, to give an information, if there is any influence of an inflammation or medication. (BE – binding efficiency, RTQ – real transport quality, DTE – detoxification efficiency)
If a patient belongs to a risk group and received a non-cancer result, we recommend an annual repetition of the albumin-functionality-test.
It is important to interpret the result of albumin-functionality-test always in connection with clinical history of the particular patient, together with results of further examinations, because beside active malignant growth also other events might result in pathological DR values – mostly only for a short time: for example infections, acute exacerbations of chronic inflammatory diseases or surgery, chemo and/or radiation therapy.
Comparison with tumor markers
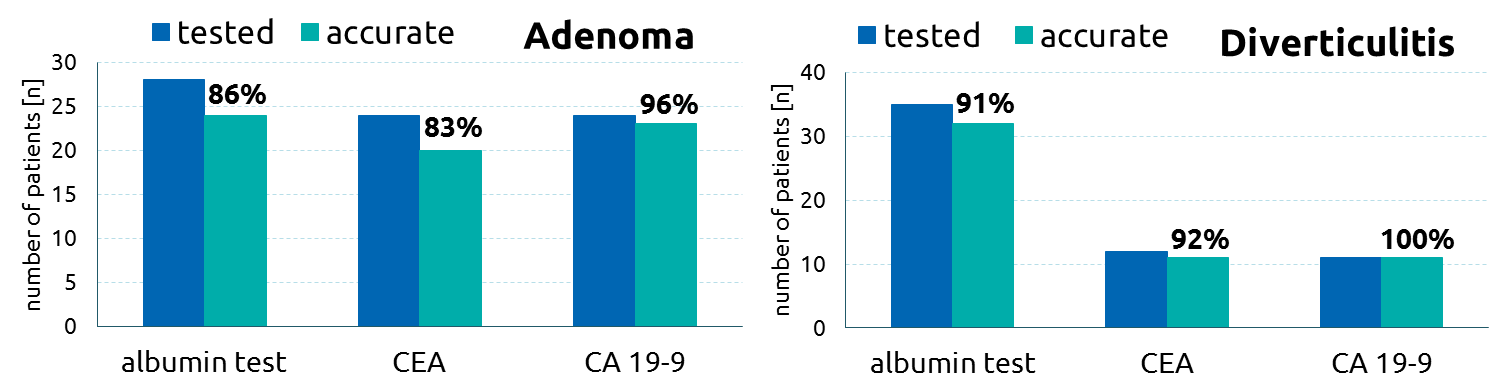
In a study investigating patients with diseases of the large bowel, the albumin-functionality-test was compared to the tumor marker CEA (Carcinoembryonic antigen) und CA 19-9.
The albumin-functionality-test showed clear advantage (91.1% accurate) compared to conventional tumor markers CEA (45.5% accurate) and CA 19-9 (27.9% accurate).

The albumin-functionality-test and the conventional tumor marker CEA and CA 19-9 are comparable in their specificity.
Publications:
Moergel M, Kammerer PW, Schnurr K, Klein MO, Al-Nawas B (2012) Spin electron paramagnetic resonance of albumin for diagnosis of oral squamous cell carcinoma (OSCC). Clin Oral Investig 16: 1529-1533. PMID: 22160580
Gelos M, Hinderberger D, Welsing E, Belting J, Schnurr K, et al. (2010) Analysis of albumin fatty acid binding capacity in patients with benign and malignant colorectal diseases using electron spin resonance (ESR) spectroscopy. Int J Colorectal Dis 25: 119-127. PMID: 19644694
Rajiv Jalan, Kerstin Schnurr, Rajeshwar P Mookerjee, Sambit Sen et al. (2009) Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. HEPATOLOGY 50: 555-564. 10.1002/hep.22913
Muravsky V, Gurachevskaya T, Berezenko S, Schnurr K, Gurachevsky A. (2009) Fatty acid binding sites of human and bovine albumins: Differences observed by spin probe ESR. Spectrochim Acta A Mol Biomol Spectrosc. PMID: 19540798
Gurachevsky A, Kazmierczak SC, Jörres A, Muravsky V. (2008) Application of spin label electron paramagnetic resonance in the diagnosis and prognosis of cancer and sepsis. Clin Chem Lab Med. 46(9): 1203-10. PMID: 18783341
Kazmierczak SC, Gurachevsky A, Matthes G, Muravsky V (2006) Electron spin resonance spectroscopy of serum albumin: a novel new test for cancer diagnosis and monitoring. Clin Chem 52: 2129-2134. PMID: 16990414
Seidel P, Gurachevsky A, Muravsky V, Schnurr K, Seibt G, et al. (2005) Recognition of malignant processes with neural nets from ESR spectra of serum albumin. Z Med Phys 15: 265-272. PMID: 16422355
Literature:
Kawashima Y, Fukutomi T, Tomonaga T, Takahashi H, Nomura F, et al. (2010) High-yield peptide-extraction method for the discovery of subnanomolar biomarkers from small serum samples. J Proteome Res 9: 1694-1705.
Taboada P, Barbosa S., Emilio Castro, Manuel Gutiérrez-Pichel, Víctor Mosquera (2007) Effect of solvation on the structure conformation of human serum albumin in aqueous–alcohol mixed solvents. Chemical Physics Volume 340: 59-68
Lowenthal MS, Mehta AI, Frogale K, Bandle RW, Araujo RP, et al. (2005) Analysis of albumin-associated peptides and proteins from ovarian cancer patients. Clin Chem 51: 1933-1945.
Simard JR, Zunszain PA, Ha CE, Yang JS, Bhagavan NV, et al. (2005) Locating high-affinity fatty acid-binding sites on albumin by x-ray crystallography and NMR spectroscopy. Proc Natl Acad Sci U S A 102: 17958-17963.
Mehta AI, Ross S, Lowenthal MS, Fusaro V, Fishman DA, et al. (2003) Biomarker amplification by serum carrier protein binding. Dis Markers 19: 1-10.
Bhattacharya AA, Grune T, Curry S (2000) Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol 303: 721-732.
Peters TJ (1995) All about Albumin: Biochemistry, Genetics, and Medical Applications Academic Press, New York
Xu Y, Shen Z, Wiper DW; et al. (1998) Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA 280(8): 719-723
He XM & Carter DC (1992) Atomic structure and chemistry of human serum albumin. Nature 358(6383): 209-215.
Reed RG, Burrington CM (1989) The albumin receptor effect may be due to a surface-induced conformational change in albumin. J Biol Chem 264: 9867-9872.
Oganesyan V, Damschroder MM et al. (2014) Structural Insights into Neonatal Fc Receptor-based Recycling Mechanisms. J Biol Chem 289:7812-7824
